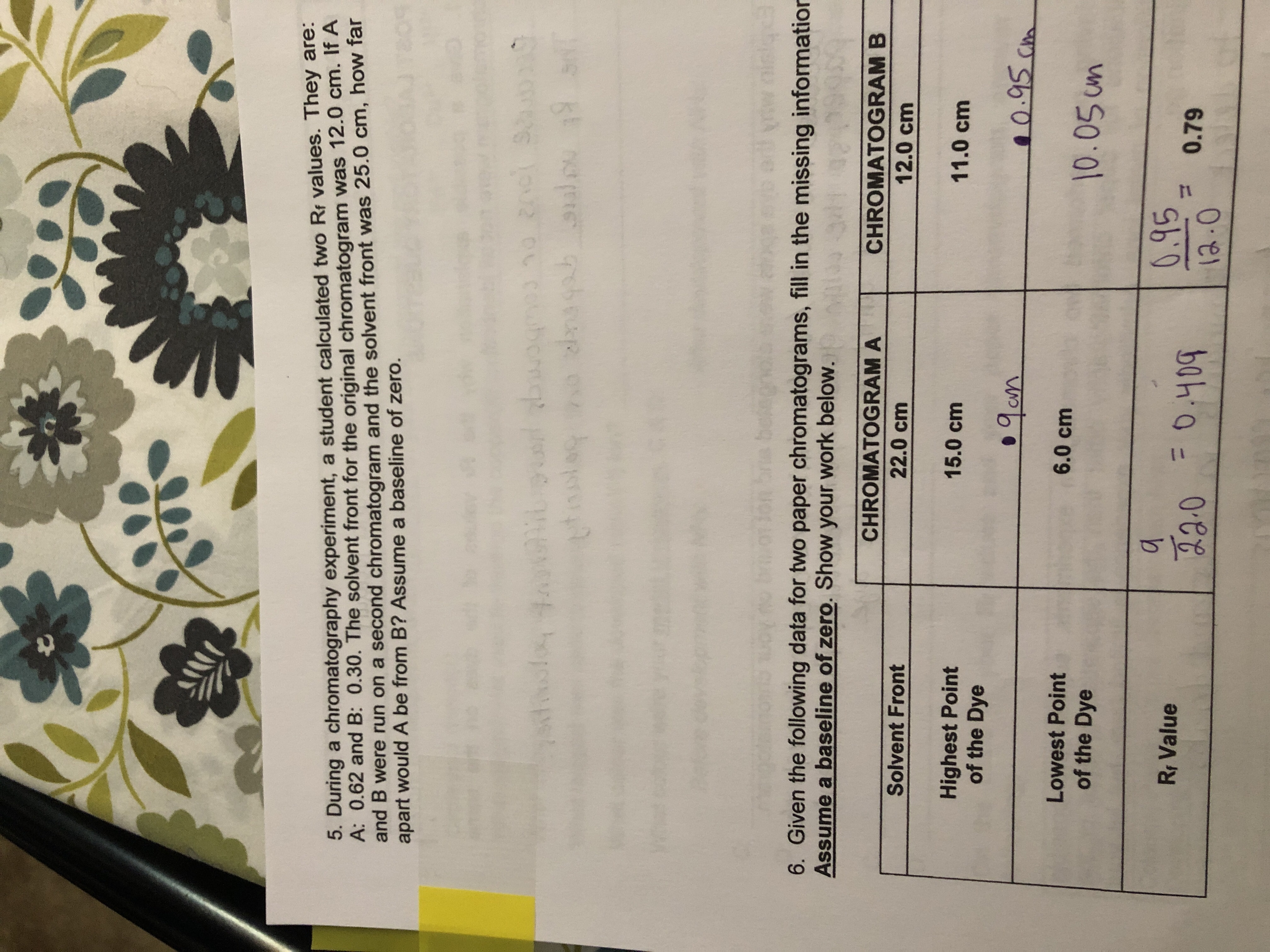
During a Chromatography Experiment a Student Calculated
If A and B were run on a second chromatogram and the solvent front was 250 cm how far apart would A be from B. On the Student Data Sheet color the diagram to illustrate the color bands on the chromatogram.

Worksheet Separation Techniques For Separating Mixtures Chemistry Worksheets Separating Mixtures Physical Science Lessons
A c 2 Een 714cm Tiff B ago 30cm 3.

. The solvent front should be 15 cm from the baseline the yellow dye should be somewhere in the region of 10 cm from the baseline and the green dye 5 cm depending on the results of throwing the dice in your experiment. Which amino acid traveled higher on the chromatography paper. Collect and distribute materials.
Describe the color of each band in Data Table 1 column B. After completing this experiment the student should be able to. Expert Answer Rf migration distance of su.
Draw a faint pencil line at the bottom of the tapered end and use a capillary pipette to add some simulated plant pigment to this line. During a chromatography experiment a student calculated two R f values. Compare Rf values to those for known substances.
In a chromatography experiment a student calculated that the solvent front was 68 cm above the starting line. PREPARATION OF CHROMATOGRAPHY PAPERS. Since the Rf value is calculated as distance spot moveddistance solvent moved the higher the Rf value the further that spot has moved.
The solvent front for the original chromatogram was 120 cm. During a chromatography experiment a student calculated two Rf values. I State the letter of the ink that contained only one coloured dye.
The goal of this lab will be to use thin-layer chromatography to determine whether a. Chromatography plate Capillary tubes 3 Amino acid solution A Chromatography solvent Hinge-topped vial Amino acid solution B Ruler UV black light Phenylalanine solution Graduated cylinder Paper towels Calculator Procedure Purpose. Assume a baseline of zero.
During a chromatography experiment a student calculated two Rf values. To the solvent front and calculate the Rf value. With a capillary tube put a small drop of amino acid on pencil line Roll up paper and stand it in a large beaker.
Flows through the stationary phase and carries the components of the mixture with it. CHEM 2211 Lab Final. It also a required practical activity in most GCSE courses table 1.
Add 5-10 ml of solvent to the reaction chamber. Use scissors to cut the bottom of the chromatography paper to a tapered end. If A and B were run on a second chromatogram and the solvent front was 250 cm how far apart would A.
During a chromatography experiment a student calculated two Rr values. Continue until all bands are labeled. The purpose of this lab was to identify the components of six unknown mixtures using thin layer chromatography and Rf value.
The solvent front for the original chromatogram was 120 cm. Paper chromatography can be used to separate the coloured dyes in inks. View the full answer Previous question Next question.
In a chromatography experiment a student calculated that the solvent front was 68 cm above the starting line. If A and B were run on a second chromatogram and the solvent front was 25cm how far apart would A be from B. What Rf values would the student calculate for arginine and glycine.
The solvent front for the original chromatogram was 120 cm. A Paper Chromatography Experiment If you have ever put a drop of liquid ink on a piece of blotting paper or filter paper and seen the different colours of ink separate you have done a chromatography experimentThe paper is a very open porous material composed of cellulose fibres. Identify the composition of an unknown drug mixture by using TLC.
The mixture is spotted on the line. A student carried out a chromatography experiment on four inks W X Y and Z. Explain basic principles of chromatography in general.
In this lab the mobile phase was in the liquid state and the compound used was ethyl acetate. If A and B were run on a second chromatogram and the solvent front was 250 cm how far apart would A be from B. A A start line is drawn near the bottom of the paper.
Chromatography is one of the most important and widely used analytical techniques known to chemists. Determine which marker was used to write the ransom note and prepare a filter paper strip for each group using that marker. The solvent front for the original chromatogram was 120 cm.
Make copies of the Reference Library pages so each group has one page for every three markers tested. Assume a baseline of zero. Measure the height of the glass jar into which the chromatography will be carried out.
The diagram shows the result. From the large sheet of chromatography paper cut a strip which is 20mm less than the height of the jar. Describe important aspects of TLC.
Arginine traveled a distance of 49 cm and glycine traveled 34 cm. Method Take chromatography paper and draw a pencil line 15cm from bottom. Chromatography is a key analytical technique students will meet several times during their secondary education see the chromatography curriculum information sheet a summary of the programme of study and subject content at key stages 3 4 and 5.
Measure the distance from the first pencil line to the solvent front. The solvent front for the original chromatogram was 120 cm. What Rf values would the student calculate for arginine and glycine.
In a chromatography experiment a student calculated an Rf value for alanine of 070 and 091 for leucine. Arginine traveled a distance of 49 cm and glycine traveled 34 cm. Students use paper chromatography and calculate the OBJECTIVES ADVANCE PREPARATION 1.
Measure the strip and cut the length to equal slightly longer than the reaction chamber. The ink is a solution of pigment light. If A and B were run on a second chromatogram and the solvent front was 25cm.
During a chromatography experiment a student calculated two Rf values. 93 n0 20c 9 19 qsbtup wogh db 444 99 6. B A beaker is filled with small amount of solvent it cannot touch or go above the start line when paper is placed in a beaker c Paper is.
The solvent in the beaker should be below the pencil line. Label the band that traveled the greatest distance 1 the next 2 the next 3. Modelling experiment a Draw on paper a half scale diagram of the chromatography paper at the end of the experiment.
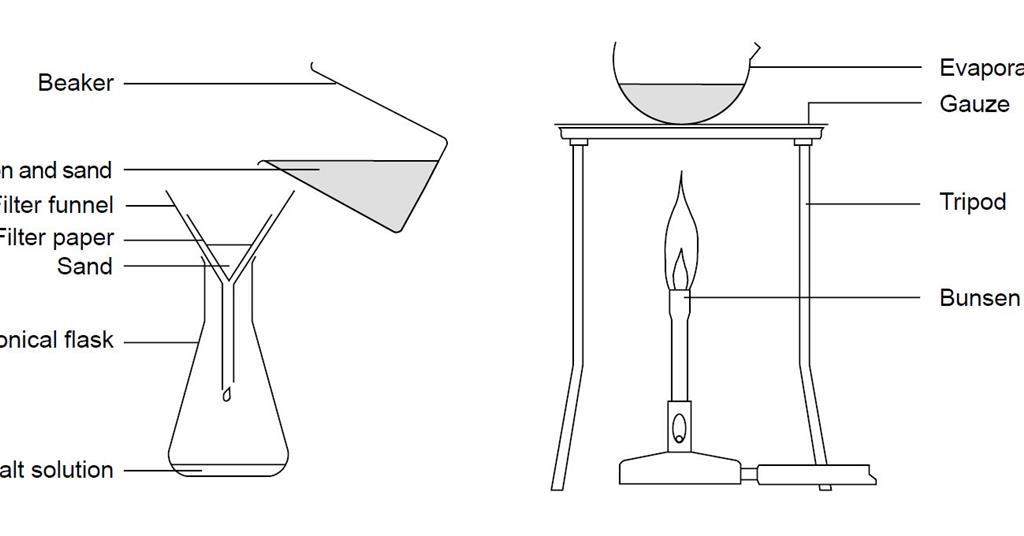
Separating Sand And Salt By Filtering And Evaporation Experiment Rsc Education

Solved Nme Date Date Tesm Section Sectinn Instructor Chegg Com
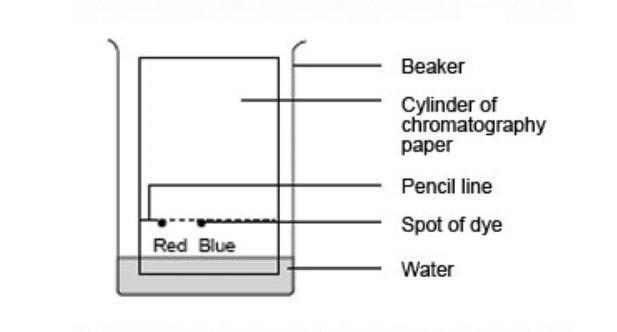
Chromatography Of Sweets Experiment Rsc Education

Leaf Chromatography Experiment Rsc Education

Learn Chemistry Socratic Chemistry Atomic Structure Atom

Answered 5 During A Chromatography Experiment Bartleby

Crossword Puzzle Naming Simple Ionic Compounds Vocabulary Review Ionic Compound Crossword Crossword Puzzle
Answered 5 During A Chromatography Experiment Bartleby
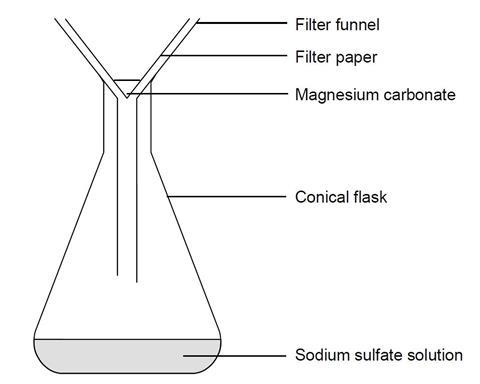
Making Magnesium Carbonate The Formation Of An Insoluble Salt In Water Experiment Rsc Education
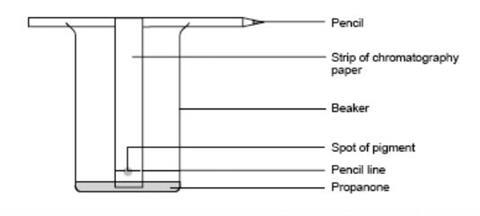
Leaf Chromatography Experiment Rsc Education
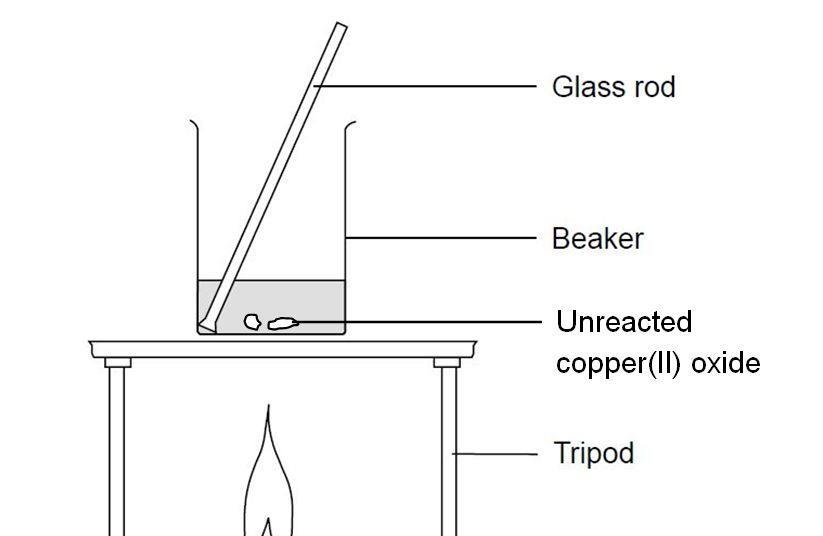
Reacting Copper Ii Oxide With Sulfuric Acid Experiment Rsc Education
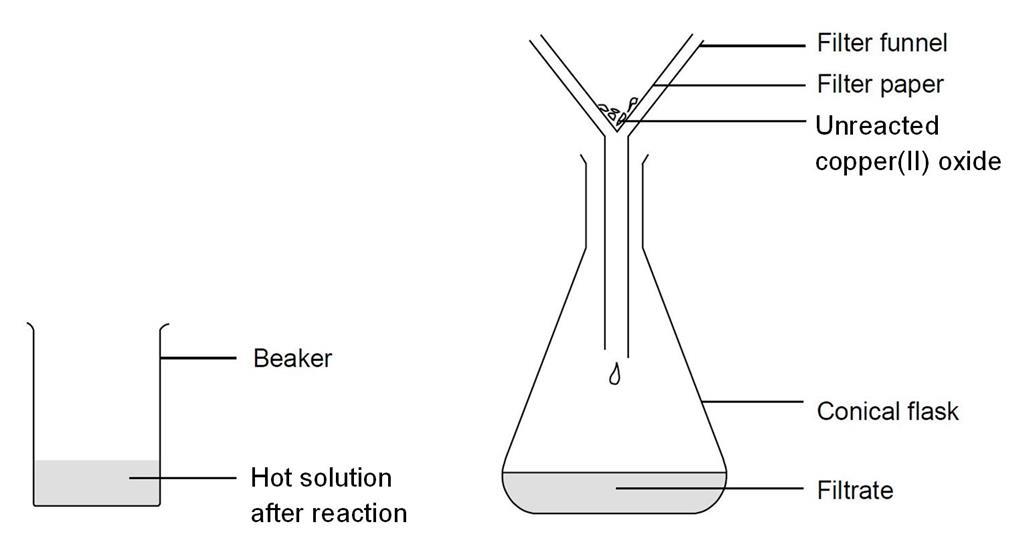
Reacting Copper Ii Oxide With Sulfuric Acid Experiment Rsc Education
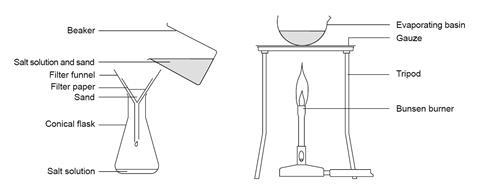
Separating Sand And Salt By Filtering And Evaporation Experiment Rsc Education

Exploring Meteorology In 2021 Lab Activities Teacher Manual Meteorology

Exploring Meteorology In 2021 Lab Activities Teacher Manual Meteorology

This Is A Lab I Use To Show Kids How The Velocity And Acceleration Of An Object Changes With The Incline O Physical Science High School Lab Activities Velocity

Race Your Marbles To Discover A Liquid S Viscosity Science Project Science Fair Projects Science Projects Science Club

Comments
Post a Comment